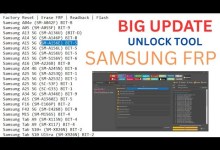
Unlocking Secrets: A Deep Dive into A24 A245F JTAG FRP Bypass Techniques!
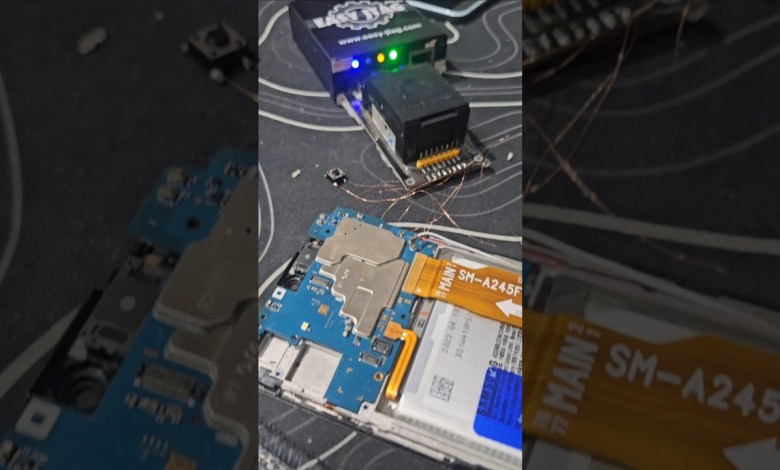
A24 A245f jtag frp bypass
Understanding Heat: The Basics and Beyond
Introduction to Heat
Heat is a form of energy that is transferred from one body to another due to a temperature difference. In our daily lives, we experience heat in various forms, whether it be the warmth of the sun, the heat from a flame, or even the warmth of a cup of coffee. Understanding heat is crucial in numerous fields, including physics, engineering, environmental science, and even culinary arts.
What is Heat?
At its core, heat refers to energy in transit. It is not contained within an object; rather, it flows from a hotter object to a cooler one until both reach thermal equilibrium. This fundamental principle is governed by the laws of thermodynamics.
The Three Types of Heat Transfer
- Conduction: This is the transfer of heat through direct contact between materials. For example, when you touch a hot stove, heat transfers from the stove to your hand.
- Convection: This occurs in fluids (liquids and gases) where heated particles move and transfer heat. Boiling water demonstrates convection; the heat from the stove heats the water, causing it to circulate.
- Radiation: This is the transfer of heat in the form of electromagnetic waves. A prime example of radiant heat is the warmth we feel from sunlight.
Measurement of Heat
The unit of measurement for heat is the joule (J) in the International System of Units (SI). In practical scenarios, particularly in cooking or home heating, we may also encounter calories and British thermal units (BTUs). Understanding these units is essential for accurate calculations in various applications.
Temperature vs. Heat
It is important to differentiate between temperature and heat. While temperature is a measure of the average kinetic energy of particles in a substance, heat is the energy transferred due to a temperature difference. Thus, an object may have a high temperature but contain less heat energy than a larger object at a lower temperature.
Real-life Applications of Heat
Heat plays an integral role in our everyday lives. From cooking food to powering engines, understanding heat is vital across various domains.
Culinary Arts
In cooking, understanding heat transfer is key to mastering techniques such as baking, grilling, and steaming. Different cooking methods rely on various types of heat transfer, from conduction in frying pans to convection in ovens.
Heating and Cooling Systems
In engineering, effective heating and cooling systems rely on the principles of heat transfer. HVAC (heating, ventilation, and air conditioning) systems are designed to regulate indoor temperatures, illustrating the practical applications of thermodynamics.
Environmental Science
Heat plays a vital role in environmental science, especially when studying climate patterns and global warming. Heat energy contributes to atmospheric and oceanic temperature changes, influencing weather patterns and ecosystems.
The Scientific Principles Behind Heat
To thoroughly understand heat, one must delve into the basic laws of thermodynamics.
The First Law of Thermodynamics
This law states that energy cannot be created or destroyed, only transformed from one form to another. When heat is added to a system, it can result in increased temperature, phase changes, or work done by the system.
The Second Law of Thermodynamics
This law introduces the concept of entropy, stating that in any energy exchange, if no energy enters or leaves the system, the potential energy available for doing work diminishes. In layman’s terms, heat naturally flows from hot to cold, not the other way around.
Heat in Advanced Physics
In advanced physics, heat is integral to discussions on thermodynamics, statistical mechanics, and quantum mechanics. Concepts such as black body radiation and heat capacity further illustrate the diverse behavior of heat under various conditions.
Black Body Radiation
A black body is an idealized physical object that absorbs all incident electromagnetic radiation. The study of black body radiation has contributed to understanding quantum mechanics and the nature of light.
Heat Capacity
Heat capacity refers to the amount of heat required to change a substance’s temperature by one degree Celsius. This concept is crucial in materials science and helps determine how different materials respond to thermal energy.
Conclusion
Heat is not just a simple phenomenon; it is a complex energy transfer process that affects multiple disciplines. Understanding the intricacies of heat is essential for scientists, engineers, and even home cooks alike. By exploring heat and its applications, we can better appreciate the natural world and utilize this knowledge in technology, art, and everyday life.
Further Reading and Resources
If you are interested in exploring more about heat, thermodynamics, and their applications, consider reading the following resources:
#A24 #A245f #jtag #frp #bypass